

|
Scientific Understanding of Consciousness |
|
|
Synaptic Vesicles Molecular Machines(Nature 490, 201–207, 11 October 2012) |
|
Nature 490, 201–207 (11 October 2012) Molecular machines governing exocytosis of synaptic vesicles Reinhard Jahn & Dirk Fasshauer Department of Neurobiology, Max-Planck-Institute for Biophysical Chemistry, 37077 Göttingen, Germany Department of Basic Neuroscience, Faculty of Biology and Medicine, University of Lausanne, 1005 Lausanne, Switzerland [paraphrase] Calcium-dependent exocytosis of synaptic vesicles mediates the release of neurotransmitters. Important proteins in this process have been identified such as the SNAREs, synaptotagmins, complexins, Munc18 and Munc13. Structural and functional studies have yielded a wealth of information about the physiological role of these proteins. Exocytosis and recycling of synaptic vesicles define how much transmitter is released from nerve terminals during incoming action potentials. Under resting conditions, synaptic vesicles are stored in the cytoplasm of the nerve terminal, with some of them attached to specialized sites at the presynaptic plasma membrane termed active zones. Active zones are composed of unique multidomain proteins that provide a scaffold for vesicle docking and participate in activating the release apparatus, referred to as priming. Priming probably involves several reactions, including some requiring metabolic energy. Docked and primed vesicles (termed readily releasable pool) are ready to go, and some do so spontaneously, with the transmitter released by a single vesicle giving rise to a miniature postsynaptic potential. When an action potential arrives, voltage-gated calcium channels open, with the resulting calcium influx stimulating the rate of exocytosis more than 100,000 fold in a highly cooperative manner. The molecular basis of synaptic exocytosis has fascinated scientists for decades. Since the initial discovery of quantal release in the 1950s by Katz and colleagues, and the elucidation of the synaptic vesicle-recycling pathway by Heuser and Ceccarelli in the 1970s, we have come a long way in deciphering the steps of the vesicle cycle at an increasingly detailed level. Although we have focused here on only a few key components, the vesicle cycle is governed by hundreds of proteins, and there are still new proteins being put on the map. We are only beginning to understand the rules by which individual protein–protein interactions work together in supramolecular machines to yield the synaptic vesicle cycle that reliably operates millions of times. These machines assemble on demand and disassemble when the task is completed. They are highly robust, tolerate varying stoichiometries, flexible compositions and other disturbances, and are controlled by an array of regulators such as protein kinases and phosphatases. Advances in technologies such as super-resolution microscopy, single-molecule measurements, fluorescent reporters and cryo-electron tomography are all contributing to closing the gap between our understanding of partial reactions in vitro and the fascinating efficiency of the vesicle cycle in intact synapses.
Return to — Neurons and Synapses |
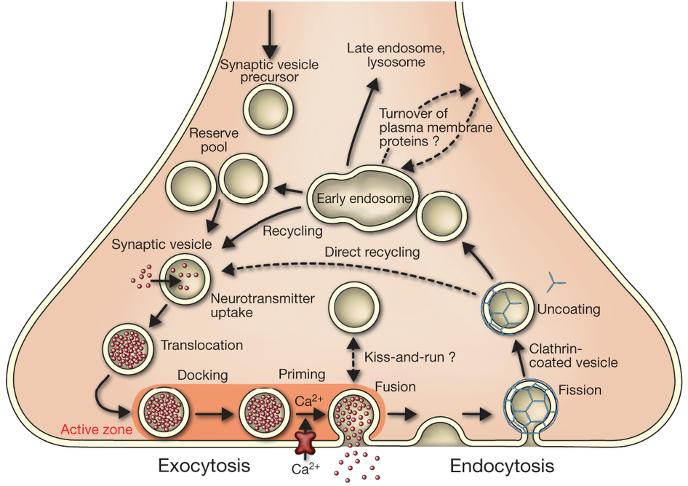
|
Trafficking pathways in the nerve terminal Synaptic vesicles are filled with neurotransmitter and stored in the cytoplasm. Active vesicles are translocated to release sites in the active zone where they dock. Priming involves all steps required to acquire release readiness of the exocytotic complex. Although usually assumed to occur after docking, priming and even triggering may precede docking during sustained activity, resulting in immediate fusion of an arriving vesicle. After exocytosis, the vesicle proteins probably remain clustered and are then retrieved by endocytosis. Despite some lingering controversies, consensus is emerging that retrieval is generally mediated by clathrin-mediated endocytosis. After clathrin uncoating, synaptic vesicles are regenerated within the nerve terminal, probably involving passage through an endosomal intermediate. Actively recycling vesicles are in slow exchange with the reserve pool. |